Feb 20, 2022Assuming that 10.0% of a 100-W light bulb’s energy output is in the visible range (typical for incandescent bulbs) with an average wavelength of 580 nm, calculate the number of visible photons emitted per second. Strategy. Power is energy per unit time, and so if we can find the energy per photon, we can determine the number of photons per
The Practical Power of Fusing Photons – IEEE Spectrum
Learn what a photon is and how to determine the energy of a photon as you explore the dual nature of light as both a wave and a particle, known as wave-particle duality. Uncover the concept of quantum mechanics, where light deposits energy in discrete amounts called photons. Investigate Planck’s constant and how it relates to the energy of a

Source Image: bartleby.com
Download Image
Step 1. Calculate the energy of a photon. E = h c λ = 6.626 × 10 − 34 J. s × 2.998 × 10 8 m. s − 1 670 × 10 − 9 m = 2.965 × 10 − 19 J Step 2: Calculate the total energy per second Total energy = 1.0 × 10 − 3 W × 1 J s − 1 1 W = 1.0 × 10 − 3 J. s − 1 Step : 3 Calculate the number of photons per millisecond. Number of photons

Source Image: byjus.com
Download Image
How Other Forms of Energy from Sunshine May Affect Our Health – GrassrootsHealth
Question Video: Calculating the Number of Photons Emitted by a Laser Given the Total Energy. A laser emits light with a wavelength of 200 nm. How many photons must be emitted by the laser for the amount of energy emitted to be 1 J? Use a value of 6.63 × 10⁻³⁴ J⋅S for the Planck constant and 3.00 × 10⁸ m/s for the speed of light in

Source Image: slideshare.net
Download Image
How To Find Number Of Photons Given Energy And Wavelength
Question Video: Calculating the Number of Photons Emitted by a Laser Given the Total Energy. A laser emits light with a wavelength of 200 nm. How many photons must be emitted by the laser for the amount of energy emitted to be 1 J? Use a value of 6.63 × 10⁻³⁴ J⋅S for the Planck constant and 3.00 × 10⁸ m/s for the speed of light in
let’s solve some numericals on quantum nature of light on photons here’s the first one we have a bulb of 100 watt 100 watt light bulb that’s giving out a light of frequency 4 times 10 to the 14 hertz now according to the quantum nature the bulb is releasing photons it’s emitting photons and so the question is how many photons are being emitted p
Chemistry (F.Sc.1) Chapter # 05 Numericals- Malik Xufyan | PDF
Jan 18, 2024Planck’s equation How to calculate the energy of a photon Energy of a photon calculator FAQ With this photon energy calculator, you can explore the relationship between the wavelength and frequency of the photon and its energy. Read the text below to find out how to calculate the energy of a photon and what is Planck’s equation. Planck’s equation
Blue Light: Should You Be Concerned?
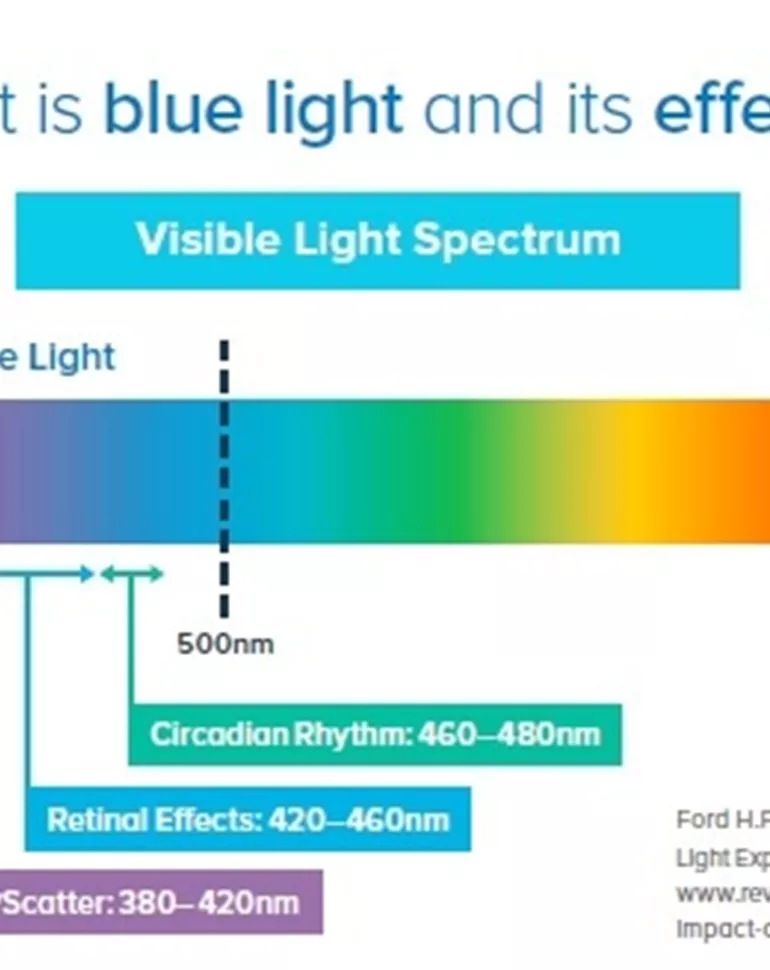
Source Image: hoyavision.com
Download Image
What is the wavelength of a photon of energy 1 eV? – YouTube
Jan 18, 2024Planck’s equation How to calculate the energy of a photon Energy of a photon calculator FAQ With this photon energy calculator, you can explore the relationship between the wavelength and frequency of the photon and its energy. Read the text below to find out how to calculate the energy of a photon and what is Planck’s equation. Planck’s equation

Source Image: youtube.com
Download Image
The Practical Power of Fusing Photons – IEEE Spectrum
Feb 20, 2022Assuming that 10.0% of a 100-W light bulb’s energy output is in the visible range (typical for incandescent bulbs) with an average wavelength of 580 nm, calculate the number of visible photons emitted per second. Strategy. Power is energy per unit time, and so if we can find the energy per photon, we can determine the number of photons per

Source Image: spectrum.ieee.org
Download Image
How Other Forms of Energy from Sunshine May Affect Our Health – GrassrootsHealth
Step 1. Calculate the energy of a photon. E = h c λ = 6.626 × 10 − 34 J. s × 2.998 × 10 8 m. s − 1 670 × 10 − 9 m = 2.965 × 10 − 19 J Step 2: Calculate the total energy per second Total energy = 1.0 × 10 − 3 W × 1 J s − 1 1 W = 1.0 × 10 − 3 J. s − 1 Step : 3 Calculate the number of photons per millisecond. Number of photons
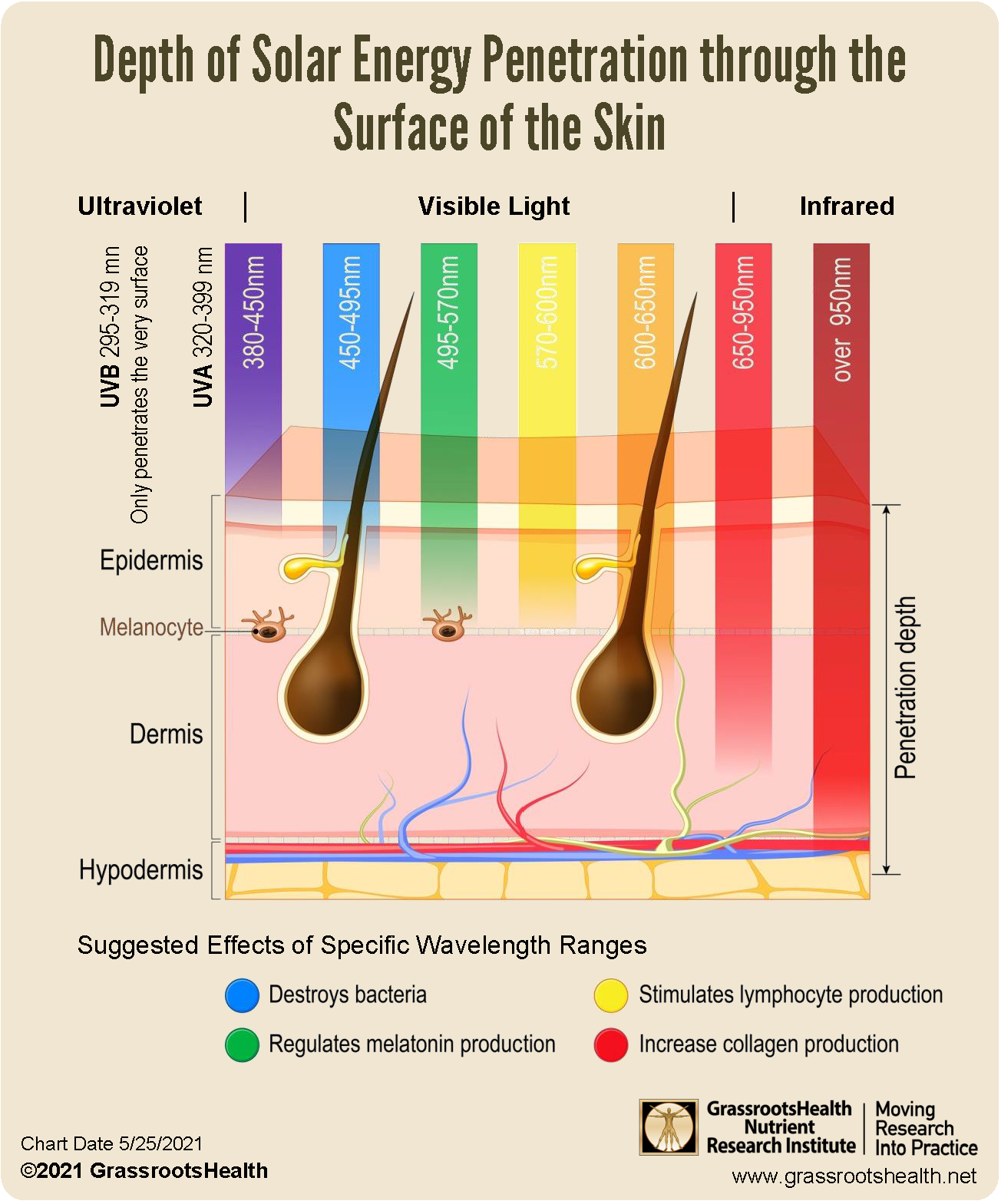
Source Image: grassrootshealth.net
Download Image
How To Calculate The Energy of a Photon Given Frequency & Wavelength in nm Chemistry – YouTube
You can use this wavelength and energy example to calculate the number of photons needed to “see”. Credit: Petr Novák, Wikipedia … You can also find the wavelength if the energy of the photon is known. … We are given the wavelength as 475 nm. Before we go any further, let’s convert this to meters. 1 nm = 10-9 m. Using this

Source Image: youtube.com
Download Image
Question Video: Calculating the Wavelength of a Photon Given Its Energy | Nagwa
Question Video: Calculating the Number of Photons Emitted by a Laser Given the Total Energy. A laser emits light with a wavelength of 200 nm. How many photons must be emitted by the laser for the amount of energy emitted to be 1 J? Use a value of 6.63 × 10⁻³⁴ J⋅S for the Planck constant and 3.00 × 10⁸ m/s for the speed of light in

Source Image: nagwa.com
Download Image
SOLVED: a.) Calculate the wavelength of a photon that would be produced when an electron jumps from the n = 5 to the n = 2 energy level in a hydrogen atom. [
let’s solve some numericals on quantum nature of light on photons here’s the first one we have a bulb of 100 watt 100 watt light bulb that’s giving out a light of frequency 4 times 10 to the 14 hertz now according to the quantum nature the bulb is releasing photons it’s emitting photons and so the question is how many photons are being emitted p
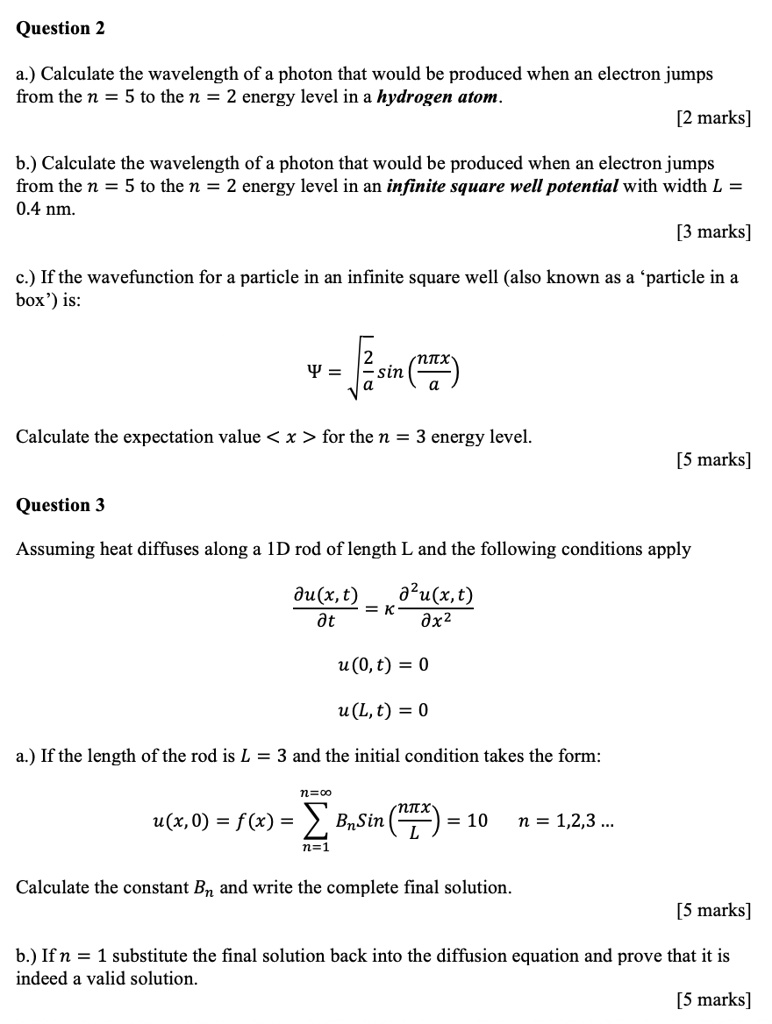
Source Image: numerade.com
Download Image
What is the wavelength of a photon of energy 1 eV? – YouTube
SOLVED: a.) Calculate the wavelength of a photon that would be produced when an electron jumps from the n = 5 to the n = 2 energy level in a hydrogen atom. [
Learn what a photon is and how to determine the energy of a photon as you explore the dual nature of light as both a wave and a particle, known as wave-particle duality. Uncover the concept of quantum mechanics, where light deposits energy in discrete amounts called photons. Investigate Planck’s constant and how it relates to the energy of a
How Other Forms of Energy from Sunshine May Affect Our Health – GrassrootsHealth Question Video: Calculating the Wavelength of a Photon Given Its Energy | Nagwa
You can use this wavelength and energy example to calculate the number of photons needed to “see”. Credit: Petr Novák, Wikipedia … You can also find the wavelength if the energy of the photon is known. … We are given the wavelength as 475 nm. Before we go any further, let’s convert this to meters. 1 nm = 10-9 m. Using this