The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled E a1 and E a2.Each elementary step has its own activated complex, labeled AC 1 and AC 2.Note that the overall enthalpy change of the reaction (Δ H) is unaffected by the individual steps, since it depends only on the initial and final states.
Draw a reaction-energy diagram for a one-step exothermic reaction… | Channels for Pearson+
Map: Organic Chemistry (Wade)

Source Image: pinterest.com
Download Image
A potential energy diagram for a two-step reaction is shown and labeled. Review What does a potential energy diagram tell us? In the diagram above, which step has the highest activation energy? If a catalyst lowered the step one activation energy to a value lower than the step two activation energy, which step would be rate-limiting?
Source Image: wou.edu
Download Image
Hybrid artificial photosynthesis technique produces hydrogen and methane Purdue University Purdue Chem 26100: Organic Chemistry I (Wenthold) Chapter 5: The Study of Chemical Reactions

Source Image: usgs.gov
Download Image
Label The Energy Diagram For A Two-Step Reaction
Purdue University Purdue Chem 26100: Organic Chemistry I (Wenthold) Chapter 5: The Study of Chemical Reactions In a reaction, any reaction, the same general trend occurs. First the bonds of the reactants are broken which requires an input of energy to be put into the reaction. This is why both diagrams have a large slope immediately following the reactants. This energy required to break all the reactant bonds is referred to as the activation energy and
The Science of Earthquakes | U.S. Geological Survey
Watch Complete videos @ www.LearningChemistryOnline.com Organic Chemistry 1 How can I draw a simple energy profile for an exothermic reaction in which 100 kJ mol-1 is evolved, and which has an activation energy of 50 kJmol-1? | Socratic
Source Image: socratic.org
Download Image
Benzoin Condensation | Organic chemistry books, Organic chemistry, Organic chemistry study Watch Complete videos @ www.LearningChemistryOnline.com Organic Chemistry 1

Source Image: pinterest.com
Download Image
Draw a reaction-energy diagram for a one-step exothermic reaction… | Channels for Pearson+ The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled E a1 and E a2.Each elementary step has its own activated complex, labeled AC 1 and AC 2.Note that the overall enthalpy change of the reaction (Δ H) is unaffected by the individual steps, since it depends only on the initial and final states.

Source Image: pearson.com
Download Image
Hybrid artificial photosynthesis technique produces hydrogen and methane A potential energy diagram for a two-step reaction is shown and labeled. Review What does a potential energy diagram tell us? In the diagram above, which step has the highest activation energy? If a catalyst lowered the step one activation energy to a value lower than the step two activation energy, which step would be rate-limiting?

Source Image: newatlas.com
Download Image
How to Draw Multi-Steps Energy Profile Diagrams: Reactant, Product, ∆H, Activation Energy, Slow Step – YouTube Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used to compare the activation energies of the different steps and identify the rate-determining step. The energy profile can also be used to determine the overall change in energy for the reaction.

Source Image: m.youtube.com
Download Image
Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition state, Delta H , and Ea. a. a concerted reaction with Delta H = -80 kJ/mol and Purdue University Purdue Chem 26100: Organic Chemistry I (Wenthold) Chapter 5: The Study of Chemical Reactions

Source Image: homework.study.com
Download Image
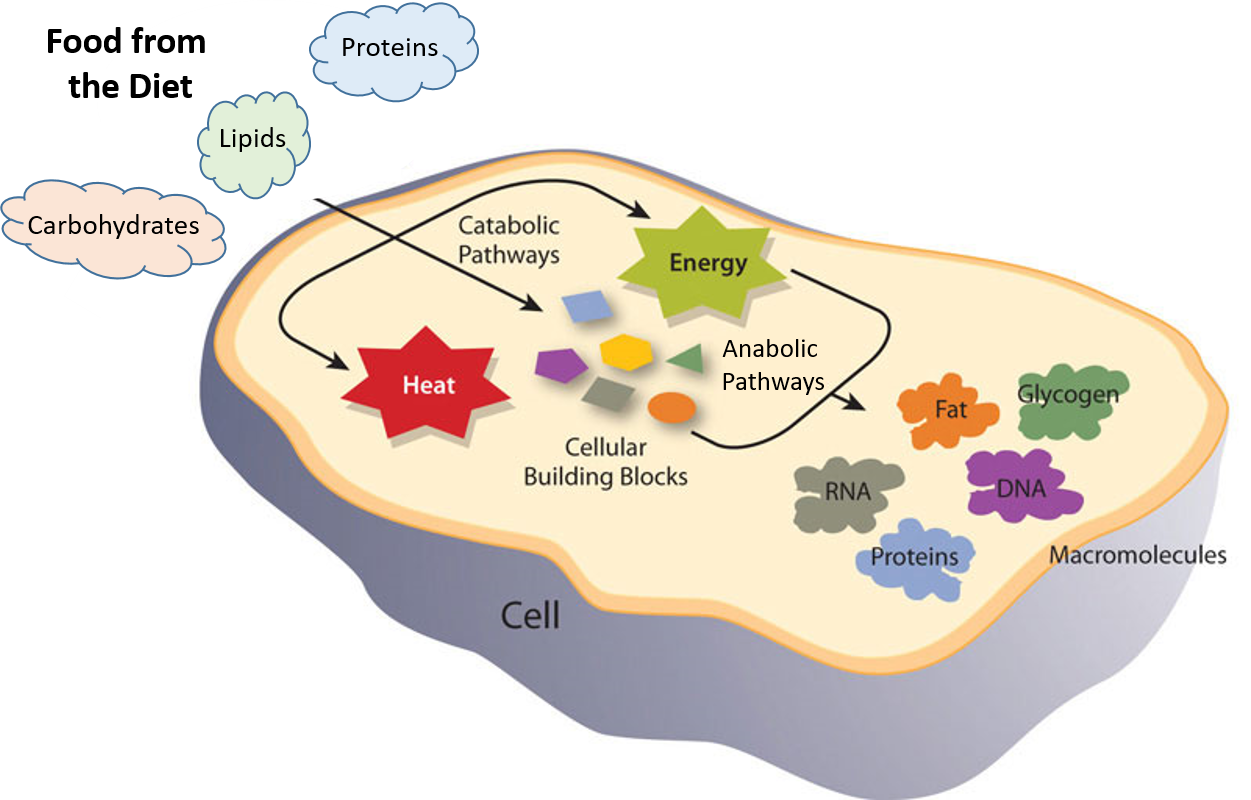
Digestion: Anatomy, physiology, and chemistry In a reaction, any reaction, the same general trend occurs. First the bonds of the reactants are broken which requires an input of energy to be put into the reaction. This is why both diagrams have a large slope immediately following the reactants. This energy required to break all the reactant bonds is referred to as the activation energy and

Source Image: medicalnewstoday.com
Download Image
Benzoin Condensation | Organic chemistry books, Organic chemistry, Organic chemistry study
Digestion: Anatomy, physiology, and chemistry Map: Organic Chemistry (Wade)
Hybrid artificial photosynthesis technique produces hydrogen and methane Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition state, Delta H , and Ea. a. a concerted reaction with Delta H = -80 kJ/mol and Many chemical reactions have mechanisms that consist of multiple elementary steps. The energy profile for a multistep reaction can be used to compare the activation energies of the different steps and identify the rate-determining step. The energy profile can also be used to determine the overall change in energy for the reaction.